Identifying and Evaluating Mediators for Electrochemical Biosensor Assays
Electrochemical biosensors are powerful tools used for detecting a wide range of analytes with high sensitivity and specificity.
Mediators are often included in electrochemical sensors as an important component that facilitates the transfer of electrons between the biological recognition element and the electrode surface. Identifying and evaluating mediators in order to pick the right mediator can significantly improve the performance of an electrochemical biosensor.

To identify a suitable mediator we must first consider the properties which are required to function well; unfortunately there is not one perfect mediator for all types of sensors.
Several key properties to consider are:
- Reversibility: The mediator should be able to undergo oxidation and reduction repeatedly without degradation
- Kinetics: Fast electron transfer kinetics are necessary to ensure a quick response time of the biosensor
- Stability: Chemical and electrochemical stability are crucial to ensure consistent performance over time
- Compatibility: The mediator should be compatible with the biological recognition element, the electrode material and test conditions (example required pH)
- Low overpotential: Often a mediator is used to lower the potential required for measurement of the analyte which helps to reduce possibility of interferences
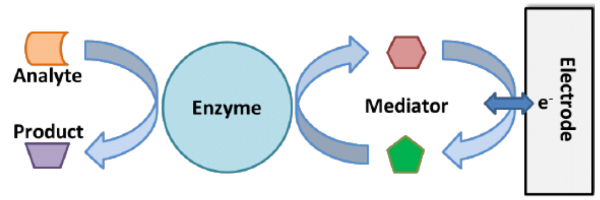
Figure 1 Shows simple diagram of enzymatic assay which uses a mediator to facilitate the transfer of electrons from the enzyme to the electrode
Identifying Potential Mediators
The initial steps of identifying potential mediators are as follows:
- Literature Review: Exploring existing research and reviews on mediators used in similar biosensing applications. This can provide insights into well-established mediators and whether their characteristics suit the requirements for the assay
- Chemical Screening: Evaluating a broad range of chemicals known for their redox properties. Common classes of mediators include ferrocene derivatives, quinones, phenazines, viologens and transition metal complexes. Conducting preliminary electrochemical tests such as cyclic voltammetry to assess the redox behavior of candidate mediators in the presence of the target analyte
Evaluating Mediator Performance
Once potential mediators are identified, they must be rigorously evaluated to ensure they meet the required performance criteria.
Key evaluation parameters include:
- Redox Potential: The redox potential of the mediator should align with the operating potential of the biosensor. Mediators with too high a redox potential may run into problems with interferences from clinical samples. A test of electrochemical properties of a given clinical matrix can be performed to understand what is potential may be suitable
- Signal Amplification: Initial optimization of electrochemical method is carried out to with the mediator. The mediator should enhance the signal generated by the biosensor. This can be evaluated by comparing the sensor’s response with and without the potential mediator candidates
- Biocompatibility: Once a base level of performance has been achieved this assay can be tested with real clinical samples. The mediator should not interfere with the clinical matrix or the analyte
- Stability: Long-term stability of the mediator is crucial for the practical application of the biosensor. This includes both chemical stability in the biological environment and electrochemical stability during repeated cycles of oxidation and reduction